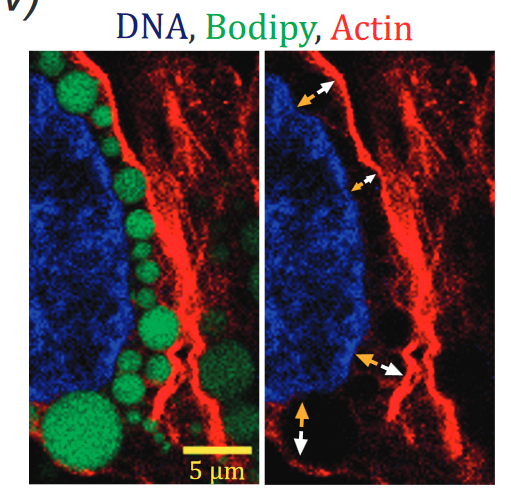
Small lipid droplets are rigid enough to indent a nucleus, dilute the lamina, and cause rupture
The nucleus in many cell types is a stiff organelle, but fat-filled lipid droplets (FDs) in cytoplasm are seen to indent and displace the nucleus. FDs are phase-separated liquids with a poorly understood interfacial tension γ that determines how FDs interact with other organelles. Here, micron-sized FDs remain spherical as they indent peri-nuclear actomyosin and the nucleus, while causing local dilution of Lamin-B1 independent of Lamin-A,C and sometimes triggering nuclear rupture. Focal accumulation of the cytosolic DNA sensor cGAS at the rupture site is accompanied by sustained mislocalization of DNA repair factors to cytoplasm, increased DNA damage, and delayed cell cycle. Macrophages show FDs and engulfed rigid beads cause similar indentation dilution. Spherical shapes of small FDs indicate a high γ, which we measure for FDs mechanically isolated from fresh adipose tissue as ∼40 mN/m. This value is far higher than that of protein condensates, but typical of oils in water and sufficiently rigid to perturb cell structures including nuclei.