Research Areas
-
Immune Checkpoints Blockade in Solid Tumors
- Nuclear Rupture Using Fat Droplets
- Cell Confinement in Solid Tumors
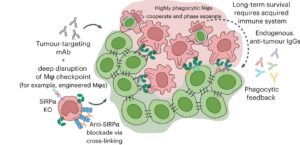
Phagocytic elimination of solid tumors by innate immune cells seems attractive for immunotherapy, particularly because of the possibilities for acquired immunity. Our lab engineers macrophages for increased phagocytic functionality by elucidating the effects different strategies can have on tumor suppression. These strategies include checkpoint blockade, chimeric antigen receptors, and polarization to clear solid tumors and induce an anti-cancer immune response.
Fat Droplets (FD’s) are phase-separated condensates which range from hundreds of nanometers to tens of microns in diameter and are found within numerous cell types. Preliminary observations indicate that FD’s dynamically interact with intracellular organelles and can apply sufficient stress to deform cell structures. We seek to uncover how the high interfacial tension and curvature of small FD’s may lead to perturbed cytoskeletal and nuclear morphologies. High resolution imaging of cancer cells demonstrates that small FD’s can indent peri-nuclear actomyosin and the nucleus, causing local dilution of Lamin-B1 and subsequent nuclear rupture and DNA damage. Similar FD-driven rupture pathways are also observed in other cell types, such as macrophages, particularly within confined microenvironments. This work aims to further elucidate the overlooked, yet physiologically disruptive, biophysical impact that FD’s have within our bodies.
Genomic instability, the inability of a cell to pass on its genetic information accurately, is a hallmark of cancer. During cancer progression, the extracellular matrix stiffens to mechanically confine cancer cells, however it is not known whether such changes in the microenvironment contribute to chromosome loss. Using hydrogel-based spheroid models, we study how extracellular matrix stiffness regulates mitotic aberrations including micronucleus formation and chromosome loss.
Solid tumors typically show higher levels of copy number variation (CNV) than that of liquid cancers, suggesting a correlation between dense microenvironments and CNV. To study this, we edited constitutive genes with GFP/RFP-tags on single alleles that function as Chromosome-Reporters (ChReporters). Thus, loss of this fluorescent signal allows us to identify individual cells that have lost a chromosome. We physically confine cells as a model for stiff tumors found in vivo, and see ChReporter loss associated with chromosome mis-segregation. Such genetic changes are shown to be heritable and a mechano-evolutionary consequence of confined microenvironments.
